Abstract
Background: Outcomes are poor for patients with large B-cell lymphoma (LBCL) who relapse early or are refractory to first-line therapy. Furthermore, patients receiving second-line standard-of-care (SOC) therapy often report poor health-related quality of life (QoL; Lin V, et al. J Clin Oncol. 2020;38:e20070). In the ZUMA-7 (NCT03391466) pivotal Phase 3, randomized, open-label, multicenter study of axi-cel (an autologous anti-CD19 chimeric antigen receptor [CAR] T-cell therapy) versus SOC, we conducted the first comparative analysis of patient-reported outcomes (PROs) with CAR T-cell therapy versus SOC as second-line treatment in relapsed/refractory (R/R) LBCL.
Methods: PRO instruments, including the EORTC QLQ-C30 (cancer-specific 30-item questionnaire including global health status, functional, and symptom scales) and the EQ-5D-5L (a general questionnaire with 5 QoL domains plus a global assessment), were administered at baseline (prior to treatment), Day 50, Day 100, Day 150, Month 9, and every 3 months from randomization up to 24 months or time of event-free survival event (disease progression, death from any cause, or new lymphoma therapy), whichever occurred first. The QoL analysis set was defined as all patients who had a baseline PRO and ≥1 measure completed at Day 50, Day 100, or Day 150. Prespecified hypotheses for 3 PRO domains (EORTC QLQ-C30 Physical Functioning, EORTC QLQ-C30 Global Health Status/QoL, and EQ-5D-5L visual analog scale [VAS]) were tested using a mixed-effect model with repeated measures at Day 100 and subsequent time points if previous time points were statistically significant. False Discovery Rate was used to adjust P values across key endpoints; sensitivity analyses were conducted to control for covariates and patterns of missingness. A clinically meaningful change was defined as 10 points for each EORTC QLQ-C30 score and 7 points for EQ-5D-5L VAS score. Exploratory analyses on other domains of EORTC QLQ-C30 and EQ-5D-5L were also performed.
Results: Of 359 patients enrolled in the ZUMA-7 study, 296 patients (165 axi-cel, 131 SOC) had baseline PROs and ≥1 follow-up measure and were included for analysis. Overall, 70% of patients had primary refractory disease, 42% had high second-line age-adjusted International Prognostic Index (2-3), and 30% were ≥65 years old.
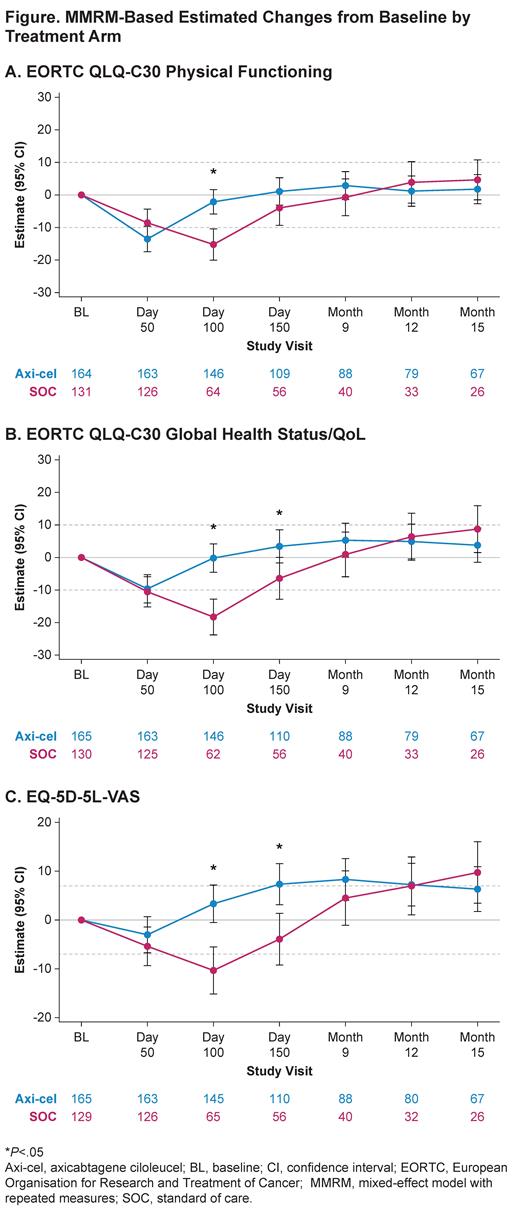
For patients in the QoL analysis set treated with axi-cel versus SOC, there was a statistically significant (P<.0001) and clinically meaningful difference in mean change of scores from baseline at Day 100 in favor of axi-cel on all prespecified PRO domains (Figure). Sensitivity analyses showed similar results with retained significance at Day 100. Furthermore, scores also significantly favored axi-cel over SOC for EORTC QLQ-C30 Global Health Status/QoL (P=.0124) and EQ-5D-5L VAS (P=.0004) at Day 150. For the prespecified endpoints, the mean estimated scores for the axi-cel arm had numerically returned to or exceeded scores at baseline by Day 150 versus on or after Month 9 for the SOC arm. After Month 9, attrition (eg, due to disease progression, new lymphoma therapy, or death) in the QoL analysis set was substantial, particularly in the SOC arm.
Additional exploratory analyses of PRO endpoints (eg, EORTC QLQ-C30 role functioning, social functioning, fatigue, nausea/vomiting, dyspnea, insomnia, appetite loss, diarrhea, and EQ-5D-5L index [US value set]) also showed improvements with axi-cel over SOC.
Conclusion: ZUMA-7, the first randomized, global, multicenter Phase 3 study of axi-cel versus SOC in second-line R/R LBCL, showed that treatment with axi-cel results in clinically meaningful improvement in QoL over SOC at Day 100 as measured by multiple validated PRO instruments. Score comparisons at later timepoints warrant cautious interpretation, particularly in the SOC arm, as attrition due to disease progression, new lymphoma therapy, or death may select patients with the best outcomes. The data also suggest faster recovery to pretreatment QoL with axi-cel compared with SOC. The superior clinical outcomes and patient experience with axi-cel over SOC should help inform treatment choices in second-line R/R LBCL.
These data are reported on behalf of all ZUMA-7 investigators and contributing Kite members.
Elsawy: Kite, a Gilead Company: Consultancy, Honoraria; Celgene/BMS: Honoraria; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; BMS: Consultancy. Chavez: MorphoSys, AstraZeneca, BeiGene, Genentech, Kite, a Gilead Company, and Epizyme: Speakers Bureau; ADC Therapeutics: Consultancy, Research Funding; MorphoSys, Bayer, Karyopharm, Kite, a Gilead Company, Novartis, Janssen, AbbVie, TeneoBio, and Pfizer: Consultancy; AstraZeneca: Research Funding; BMS: Speakers Bureau; Merk: Research Funding. Avivi: Novartis: Speakers Bureau; Kite, a Gilead Company: Speakers Bureau. Larouche: Gilead: Consultancy. Wannesson: Novartis: Consultancy, Research Funding; MSD: Consultancy; BMS: Consultancy; AstraZeneca: Consultancy; Roche: Consultancy, Research Funding. Cwynarski: Kite, a Gilead Company: Consultancy, Other: travel to scientific conferences, Speakers Bureau; Takeda: Consultancy, Other: travel to scientific conferences, Speakers Bureau; Celgene: Consultancy; Atara: Consultancy; Gilead: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; BMS/Celgene: Other: travel to scientific conferences; Janssen: Consultancy, Other: travel to scientific conferences; Roche: Consultancy, Other: travel to scientific conferences, Speakers Bureau. Osman: Kite, a Gilead Company: Consultancy. Davison: Merck: Consultancy; AstraZeneca: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Gilead: Consultancy; Abbvie: Consultancy; Celegene: Consultancy. Rudzki: Kite, a Gilead Company: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau; Amgen: Consultancy; MSD: Consultancy; Roche: Consultancy, Speakers Bureau; BMS-Celgene: Consultancy. Dahiya: Jazz Pharmaceuticals: Research Funding; Miltenyi Biotech: Research Funding; Kite, a Gilead Company: Consultancy; Atara Biotherapeutics: Consultancy; BMS: Consultancy. Dorritie: OncLive/Institutional Perspectives on Cancer presentation: Honoraria; Janssen: Research Funding; University of Pittsburgh/UPMC Hillman Cancer Center: Current Employment; Juno/BMS: Research Funding; F. Hoffman-La Roche Ltd: Research Funding; Kite, a Gilead Company: Research Funding; Genmab: Research Funding; SITC presentation: Honoraria. Jaglowski: CRISPR Therapeutics: Consultancy; Takeda: Consultancy; Juno: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Radford: AstraZeneca: Current holder of individual stocks in a privately-held company; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; ADC Therapeutics: Consultancy, Current holder of individual stocks in a privately-held company, Honoraria, Speakers Bureau; BMS: Honoraria. Morschhauser: Servier: Consultancy; Roche: Consultancy, Speakers Bureau; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Chugai: Honoraria; Genmab: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech, Inc.: Consultancy; Janssen: Honoraria; Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZenenca: Membership on an entity's Board of Directors or advisory committees. Cunningham: AstraZeneca: Research Funding; Clovis Oncology: Research Funding; MedImmune: Research Funding; Roche: Research Funding; Celgene: Research Funding; Bayer: Research Funding; 4SC: Research Funding; Eli Lilly: Research Funding; OVIBIO: Membership on an entity's Board of Directors or advisory committees. Martin Garcia-Sancho: Kern Pharma: Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Incyte: Consultancy; Takeda: Honoraria; Novartis: Consultancy; Eusa Pharma: Consultancy; Clinigen: Consultancy; Kyowa Kirin: Consultancy; Morphosys: Consultancy; Gilead: Consultancy, Honoraria; Servier: Consultancy, Honoraria, Other: Travel/Accommodations/Expenses; Janssen: Honoraria, Research Funding; Celgene/BMS: Consultancy; Celgene: Honoraria, Other: travel; Roche: Consultancy, Honoraria, Other: Travel/Accommodations/Expenses. Tzachanis: Partner: Consultancy; Takeda: Consultancy, Speakers Bureau; EUSA: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding, Speakers Bureau; Kyowa Kirin: Consultancy; Magenta: Consultancy; Bristol Myers Squibb: Research Funding; Genentech: Research Funding; Incyte: Research Funding; Fate Therapeutics: Research Funding. Karmali: Roche: Consultancy; BMS/Celgene/Juno: Consultancy, Research Funding; EUSA: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding, Speakers Bureau; Morphosys: Consultancy, Speakers Bureau; Epizyme: Consultancy; Janssen/Pharmacyclics: Consultancy; AstraZeneca: Speakers Bureau; Genentech: Consultancy; Karyopharm: Consultancy; BeiGene: Consultancy, Speakers Bureau; Takeda: Research Funding. Kekre: Gilead: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria. Thieblemont: Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses , Research Funding; Bristol Myers Squibb/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Kyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Cellectis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Hospira: Research Funding; Bayer: Honoraria; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses . Enblad: Kite, a Gilead Company: Consultancy; Gilead Sciences: Consultancy. Dreger: Riemser: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Bluebird Bio: Consultancy; Janssen: Consultancy; Gilead Sciences: Consultancy, Speakers Bureau; Roche: Consultancy, Speakers Bureau; BMS: Consultancy; AstraZeneca: Consultancy, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau. Malladi: Gilead: Honoraria, Other: Travel support; Gilead Science: Consultancy. Joshi: Open Health: Current Employment; Kite, a Gilead Company: Consultancy, Research Funding; various clients via employment: Consultancy, Research Funding. Wang: Kite, a Gilead Company: Consultancy, Research Funding; additional companies through employment with Open Health: Consultancy, Current Employment, Research Funding. Solem: OPEN Health: Current Employment; Kite, a Gilead Company: Consultancy; multiple clients through employment at OPEN Health: Research Funding. Thornton Snider: Kite, a Gilead Company: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Gilead: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. To: Kite, a Gilead Company: Current Employment, Other: stock or other ownership ; NantWorks: Ended employment in the past 24 months. Kersten: BMS/Celgene: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Other: Travel support, Research Funding; Novartis: Consultancy, Honoraria, Other: Travel support; Miltenyi Biotec: Consultancy, Honoraria, Other: Travel support; Celgene: Research Funding; Takeda: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel support, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal